step 1.参考文献
1941年在猫薄荷中分离出荆芥内酯(JACS.1941.63.1558),67-80%猫猫会对其起反应。

合成路线如下:
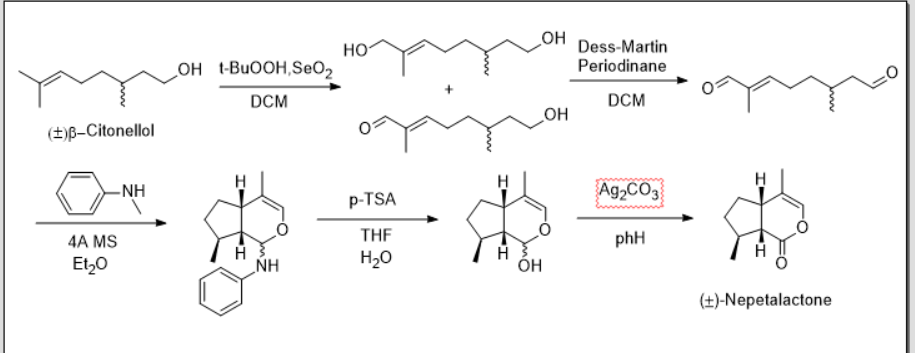
(绘图软件:Chemdraw)
Step 1.二氧化硒氧化氧化烯丙基位的C-H得到烯丙醇产物
反应机理
[2,3]σ重排(Ref:J. Org. Chem. 2000, 65, 7554., Tetrahedron Lett.2003, 44, 1099.)

虽然这试剂是有毒的,但是该反应非它不可,条件也很温和所以经常还是在被使用。如果加入助氧化剂TBHP的话,可以把二氧化硒的使用量减少到催化量。
step1.

操作步骤:
Racemic citronellol (23.4 mL, 128 mmol, 1 eq) was added to a solution of SeO2 (0.855 g, 7.7 mmol, 0.06 eq), salicylic acid (2.13 g, 15.4 mmol, 0.12 eq), and t-BuOOH (70% in H2O, 53 mL, 553 mmol, 4.3 eq) in DCM (40 mL) and the resulting reaction mixture was stirred at rt for 72 h. The reaction mixture was diluted with toluene (100 mL) and the volatiles were removed by rotary evaporation. The residue was diluted with ether (400 mL) and washed with NaOH (10% w/v, 4 × 140 mL) and brine (1 × 120 mL). The organic layer was dried over MgSO4 (anhyd), filtered, and the volatiles were removed by rotary evaporation to yield a clear yellow oil (21.2 g, 95%).
原料香茅醛
![M]L7O64DSR1UE($~@6}F%EE.jpg](https://img.kechuang.org:81/r/324237?c=resource)
Racemic citronellol (23.4 mL, 128 mmol, 1 eq)in DCM (40 mL)

SeO2 (0.855 g, 7.7 mmol, 0.06 eq)& salicylic acid (2.13 g, 15.4 mmol, 0.12 eq)、

t-BuOOH (70% in H2O, 53 mL, 553 mmol, 4.3 eq)

The mixture was stirred at r.t. for 72 h

After 3 days,analysis of the reaction mixture by TLC(Hexane:ethyl acetate=5/1)
![XE}FYGJY0Y{]%OVONMP_DYA.jpg](https://img.kechuang.org:81/r/324476?c=resource)
由于最近莫得时间,反应体系放置了一周之久。
After 7 days,analysis of the reaction mixture by TLC(Hexane:ethyl acetate=2/1)

未完待续,慢更

[修改于 3年2个月前 - 2021/09/24 17:47:29]
引用孙益楠_BG5BNA发表于11楼的内容是复现B站上那个吗XXXXXXXXXXXXXXXXXXXXXXXX/video/BV15s411R7...
不是的,第一步改为一锅法两步氧化。提高收率。两步氧化收率比香茅醛一步高,因为二氧化硒氧化本身就是产烯丙醇的,那个烯丙醛是过度氧化产物。这里会产生醇和醛然后再一锅氧化成二醛(避免用香茅醛时的过度氧化)。然后环化和水解变成一锅法,提高TsOH用量,这样能更简单一些。这个改进方法同共只有三步(包括两步一锅法合成),第一步:二氧化硒氧化后戴斯马丁氧化剂进一步氧化成二醛,第二步:形成亚胺后杂D-A反应合环然后水解,第三步:氧化缩醛得到内酯(用NMO配伍TBAP水系也行,但是TBAP是高钌酸盐很贵)
引用20!Dopaminor发表于13楼的内容怎么用,用甘油稀释?稀释到什么浓度?一次多少毫克?
酒精稀释可以当香水,要是用于猫猫是极少量酒精稀释后在水中分散。浓度是很稀的就可以
最近莫得时间,反应物一直放在那里。竟然意外发现,烯丙醇过度氧化成为我要的烯丙醛。反应三天时明显发现有两个点,反应七天后只剩小极性的点。
TLC如下(石油醚/乙酸乙酯:2/1)

After 3 days,the reaction mixture was diluted with toluene (100 mL) and the volatiles were removed by rotary evaporation. The residue was diluted with ether (400 mL) and washed with NaOH (10% w/v, 4 × 140 mL) and brine (1 × 120 mL). The organic layer was dried over MgSO4 (anhyd), filtered, and the volatiles were removed by rotary evaporation to yield a clear yellow oil。Then Dess-Martin periodinane was added to a solution of this product in DCM and the resulting reaction mixture was stirred at 0℃ overnght。

| 时段 | 个数 |
|---|---|
| {{f.startingTime}}点 - {{f.endTime}}点 | {{f.fileCount}} |
200字以内,仅用于支线交流,主线讨论请采用回复功能。